Author: Jeff Pawelek –
Digital trials have the capacity to improve access for underrepresented communities to participate in research, identify novel digital endpoints to expand our understanding of health outcomes, and elevate the level of engagement and overall experience for study participants. However, navigating and implementing these capabilities can be a challenge for research professionals. In light of this, professional organizations have developed digital trial tools and resources for the research community. In this blog, we take a snapshot of some of the tools developed by Clinical Trials Transformation Initiative (CTTI), Digital Medicine Society (DiMe), and Decentralized Trials & Research Alliance (DTRA).



Digital tools to boost diversity in research
It’s clear that improving representation in clinical research studies and clinical trials is essential to improving public health. Last year, the FDA released draft guidance on this important topic (“Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry”), and in support of this critical mission, several organizations have shared their best practices and recommendations.
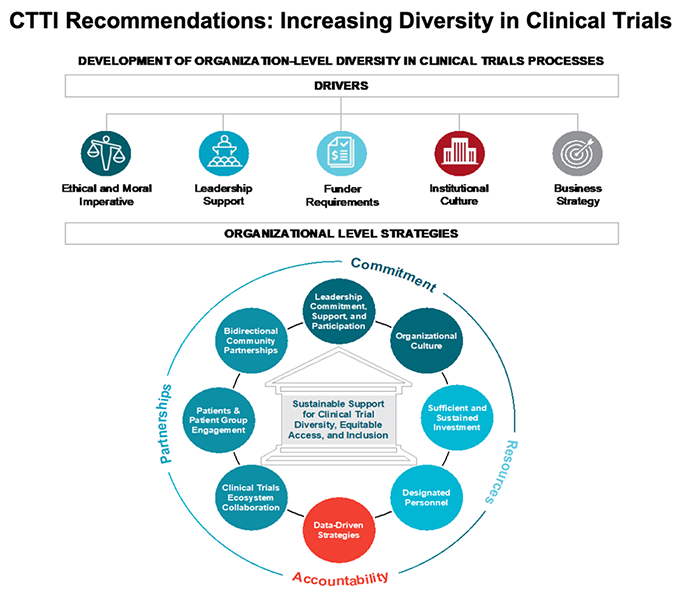
To help investigators develop diversity, equity and inclusion (DEI) initiatives into their research portfolio, CTTI has published a guidance document with recommendations on how to implement sustainable DEI practices as well as a Diversity Maturity Model to help organizations navigate their DEI needs based on their existing infrastructure and future goals. Additionally, earlier this year, DiMe posted their own resources directed at improving DEI in digital trials. This effort was in collaboration with Scripps Research, US Food and Drug Administration, National Minority Health Association, American Kidney Fund, and several corporate partners. DiMe’s DEI resource consists of standardized definitions, guidance on choosing the right digital tool at specific stages of a digital trial, and how to use these tools to improve enrollment and retention of underrepresented study participants.
Choosing the right digital measures and study endpoints
Among CTTI’s and DiMe’s range of resources, there is particular focus on digital endpoints in decentralized trials. CTTI’s “Developing Novel Endpoints” is geared towards investigators who wish to select endpoints that have real-world meaning and clinical relevance to study participants and health care providers. This resource addresses technological and regulatory considerations for both observational and interventional studies. Specific use cases for a variety of health conditions are included to contextualize the information for investigators.
DiMe’s digital endpoint library is a crowdsourced reference of digital measures used in industry-supported studies. The library is a living repository of new and existing digital health products, what they measure, how they’re being studied, and for what health conditions. The library contains roughly 400 distinct endpoints that have been used in device and drug clinical trials, along with use cases that demonstrate how industry has leveraged this resource to support their research efforts.

Optimizing the participant experience
All three organizations (CTTI, DiMe, DTRA) have contributed to the development of best practices for digital trial stakeholders, specifically when it comes to participant engagement and their overall study experience. CTTI partnered with the FDA to create the Patient Engagement Collaborative, which connects a diverse community of patients directly with the FDA to discuss the patient role in developing medical products and regulatory guidance. DiMe developed The Playbooks, a comprehensive framework of digital health resources including the Digital Healthcare Edition that was created in partnership with the Veterans’ Health Administration. This edition has a dedicated section on digital engagement tools including the use of social media to advance patient education. Of note, Scripps Research was a collaborator and contributor to The Playbooks.
DTRA developed a rubric aimed at evaluating the effectiveness of practices that drive the lifecycle of a digital study. The rubric was designed to help investigators determine whether a specific strategy can be considered a “best practice” and includes ways to measure methods that are aimed at improving patient engagement and the overall study experience.

Just the beginning
There are many more resources offered by these organizations, such as CTTI’s best practices for creating master protocols, DiMe’s course on ethics in digital health, and DTRA’s glossary of industry terms and definition. As industry, academia, government agencies, and hospitals continue to adopt digital health solutions to support their clinical research goals, the demand for proven tools and resources will continue to grow to realize the full potential of digital clinical trials.



