Authors: Lauren Serpico & Jeff Pawelek –
In Part 1 of this series, we explored the digital recruitment strategies that are designed to engage a diverse community of individuals to participate in cutting-edge research studies. From the power of strategic partnerships to social media outreach, we explored the emerging strategies that are driving digital engagement.
In Part 2 of this series, we’ll discuss how to achieve protocol adherence (to reduce data missingness) and the significance of long-term participant retention. We will share some evidence from the academic literature and share some of our own successful use cases that have led to enduring participant engagement.

Engagement through in-app learning
Educating participants about the research study topic or the health condition under investigation is a core strategy in the toolkit of long-term engagement. It’s more than just data collection, it’s about ensuring that participants understand the broader context of their involvement and the potential impact of their contribution. By offering convenient and relevant health information, participants can gain a deeper appreciation for the study’s significance and their role in advancing medical knowledge. Informed participants may be more likely to stay engaged throughout the trial, as they recognize the real-world implications and potential benefits of their participation.
For example, as mentioned in a previous blog article, with an aim to improve participant knowledge and engagement, we integrated MedlinePlus Connect in our participant-facing study apps (Figure 1). MedlinePlus Connect is a trusted health information resource that offers a consumer-focused library and dictionary. By offering this utility within our study apps, study participants can search for the latest health information without having to leave the app. This resource provides a convenient way for participants to learn about various aspects of the study including questions about their own health.

Figure 1. Participant-facing screenshots showing before and after the integration of MedlinePlus Connect.
Return of real-time, personalized information
The vast majority of people who participate in traditional clinical trials experience a one-way transaction with respect to the flow of information. In most studies, participants share their personal and health-related information with researchers without expecting an interpretation of their personal study results. We believe that consistent, personalized and actionable return of results to participants can lead to more meaningful engagement, resulting in more participants remaining engaged until the study’s completion. The return of results can contextualize personal study data in a way that is meant to empower participants with information that can help inform future discussions with their health care providers. This may foster a sense of partnership: investigators providing value to participants while also gathering critical information that can help answer questions that can positively impact public health.
For example, our REFRESH study (REsearch FRamework Exploring Sleep Health), we designed reports for study participants that show their personal survey results, providing real-time feedback and individual-level information that may help improve their own knowledge about their health and inform future discussions with their healthcare providers (Figure 2). In another study (PRediction Of Glycemic RESponse Study or PROGRESS), participants are able to track their individual glucose levels in real-time before and after eating a meal using the study app (connected via their continuous glucose monitor) (Figure 3).


Figure 2. Participant-facing screens showing personalized return of survey results for the REFRESH study.
Point-based incentives and in-app messaging
For PROGRESS, participants were asked to complete multiple complex tasks such as filling out surveys, collecting their own biosamples, and connecting wearable devices and sensors. We designed a point-based incentive system with in-app messaging to boost adherence throughout the study period. As participants completed specific milestones, they earned points which could be redeemed for gift cards. Major study milestones were weighted so participants could earn more points, which incentivized them to complete tasks at critical stages of data collection. Using a game-like experience, once a participant completed a study milestone the next threshold of study tasks was unlocked with in-app messaging that encouraged them to continue their study journey (Figure 3).
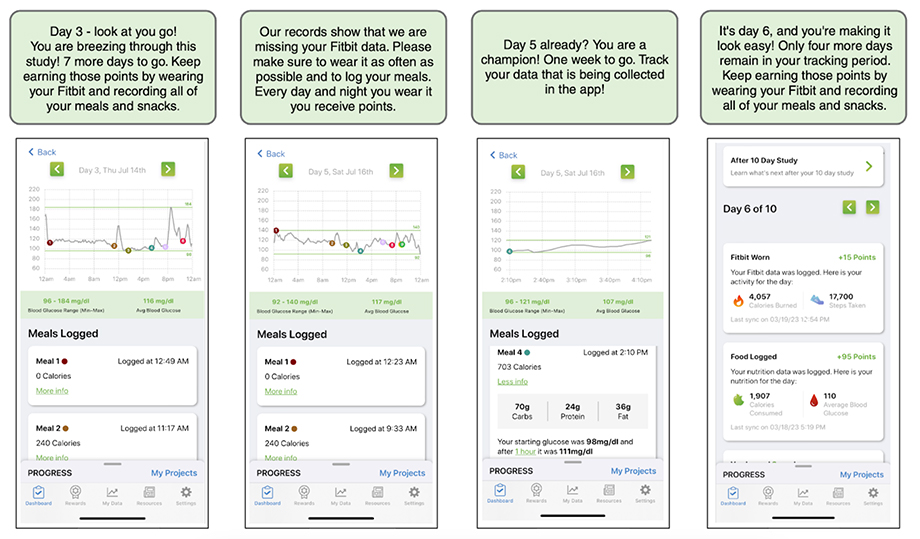
Figure 3. Screenshots from the PROGRESS app that provide feedback and motivation to study participants. The app displays the study tasks that the participants have completed, their real-time glucose levels from wearable sensors, and the points they have earned for their efforts. The app also sends timely encouragements to the participants to help them stay on track to complete the study.

The right digital cues at the right time
Just-in-time reminders, or real-time prompts, can significantly improve participant adherence and retention1. By providing reminders, valuable information, and support precisely when participants need it most, these interactions bridge the gap between the participant’s daily life and fulfilling their commitment to the study.
The strategy of assigning study tasks on the very day of consent can also improve adherence to study tasks over time. This approach ensures that participants embark on their trial journey with immediate engagement, reducing the risk of procrastination or disengagement.

To learn more about the Scripps Research Digital Trials Center and our ongoing research efforts, please visit our website.
References
- Schembre SM, Liao Y, Robertson MC, Dunton GF, Kerr J, Haffey ME, Burnett T, Basen-Engquist K, Hicklen RS. Just-in-Time Feedback in Diet and Physical Activity Interventions: Systematic Review and Practical Design Framework. J Med Internet Res. 2018 Mar 22;20(3):e106. doi: 10.2196/jmir.8701. PMID: 29567638; PMCID: PMC5887039.



