Author: Lauren Serpico –
As the new year draws near, we reflect on the significant advancements made in digital clinical trials in 2023. Our hope in sharing these reflections is to expand the scope for greater participation and benefit within biomedical research in the new year and beyond.
In this post, we share what we have deemed the top five innovations in digital clinical trials over the past year. With examples from our studies and scientists, driven by wearable technologies, artificial intelligence integration, and personalized participant engagement, these advancements signify another leap forward in fostering a collaborative and technologically enriched future for biomedical research.

Rapid expansion of novel wearable devices and remote sensors
New wearable devices and remote sensors have significantly broadened the scope of data collection in biomedical research. Innovative technologies, such as smart cardiac scales and vital sign patches, go beyond traditional health-related clinical measures. Smart cardiac scales not only measure body weight, but also offer insights into critical heart function parameters such as peripheral impedance, pulse rate, and hemodynamic biomarkers. Vital sign patches gather a diverse array of data including heart rate, heart rate variability, respiratory rate, physical activity levels, and continuous single lead electrocardiogram readings.
“Most people, when they think about sensors, they’re thinking about something like a smartwatch or a fitness band and just the data that you can get from that like heart rate. There’s also, of course, chemical sensors where you can get something like glucose. But the sensor that gets me most excited is actually imaging. So here you’re taking a probe that gets high resolution ultrasound and you connect it to the base of a smartphone. And what’s amazing about it is you don’t even have to know what you’re doing. All you have to do is be able to put the probe somewhere close to the organ of interest. And the AI will tell you, you know, move it up, move it down, move it clockwise, counterclockwise, and it automatically captures the image of interest. And now we’re seeing automatic interpretations that are really good for screening. So this is a revolution that’s just getting legs right now. But the implications of it are vast.” – Eric Topol, MD
AI is taking the digital revolution of health research to the next level
AI is broadly defined as the discipline devoted to the development of technologies to empower machines with capabilities that are typical of human intelligence. An area where we are experiencing a digital revolution in health research with AI is data analysis.
The availability of massive and detailed health datasets enabled the use of AI to solve challenging clinical problems that would have been unthinkable only a decade ago. Machine Learning is used to create algorithms that are able to extract patterns and rules to make predictions without being explicitly programmed to do so. Our scientists at the Scripps Research Digital Trials Center are now leveraging AI-powered analytics throughout our precision medicine research portfolio
A new AI model designed by our scientists could help clinicians better screen patients for atrial fibrillation (or AFib)—an irregular, fast heartbeat that is associated with stroke and heart failure. The model picks up on tiny variations in a person’s normal heartbeat that signify AFib risk, which standard screening tests cannot detect. The findings, described in the journal npj Digital Medicine used data on nearly half a million people who had each worn an electrocardiogram (ECG) patch to record their heart rhythms for two weeks—a routine screening test for AFib and other heart conditions. An AI model then analyzed this data to find patterns, other than AFib itself, that distinguished people with AFib from those without. This new model holds the potential to better detect those who are at risk for AFib and ultimately prevent this heart condition’s severe side effects, including stroke and heart failure.
Using well-known technologies for unique applications
Clinical research often has a population-level approach, which is important to ensure we are developing new treatments at scale. This approach often has the unintended consequence of sub-populations waiting for answers more specific to their underlying conditions. Given the unique health risks of immunocompromised individuals and those over 65, our scientists recognize the importance of considering them in the context of COVID-19 infection.
In our ImmunoCARE study, we are exploring whether repeated at-home tests, on-demand telemedicine, and quick delivery of medication for those who test positive for COVID-19 can reduce symptom severity in immunocompromised people and those over 65.
“Another example of how wearables can give a more complete picture of someone’s health is conditions like long-COVID, where what happens is you have something called post-exertional malaise. So you exert yourself and then you have a symptom exacerbation. But a lot of times there’s a time lag in that. It could be a few hours, it could be several days. And so when you’re at the doctor’s office, a lot of folks with long-COVID can present as relatively normal, but then they completely crash for a week after that just by the exertion of going to the doctor’s office. Therefore, wearables that can give a more complete picture of functioning can show that sort of a more full picture of how someone is really presenting in their health, rather than just that small slice that you get at the doctor’s office.”
The power of returning information to participants
The vast majority of people who participate in traditional clinical trials experience a one-way transaction with respect to the flow of information. In most studies, participants share their personal and health-related information with researchers without expecting an interpretation of their personal study results. We believe that consistent, personalized and actionable return of results to participants can lead to more meaningful engagement between researchers and the participant community.
Our PRediction Of Glycemic RESponse Study (PROGRESS) explores type 2 diabetes and glycemic response through a precision nutrition lens with a sophisticated combination of participant-reported outcomes, fitness tracker metrics, self-guided biosample collections, and novel, personalized data visualizations for participants.
Eric Topol, MD, notes PROGRESS as “one of the most exciting trials ever done digitally.” In this study, participants are able to track their individual glucose levels in real-time before and after eating a meal using the study app (connected via their continuous glucose monitor).
Return of results can contextualize personal study data in a way that is meant to empower participants with information that can help inform future discussions with their health care providers. This may foster a sense of partnership: investigators providing value to participants while also gathering critical information that can help answer questions that can positively impact public health.
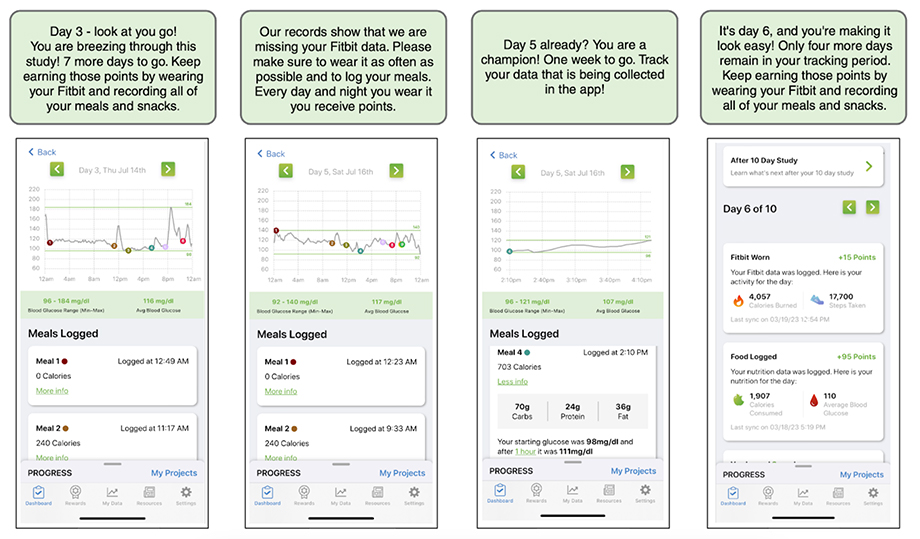
Screenshots from the PROGRESS app that provide feedback and motivation to study participants. The app displays the study tasks that the participants have completed, their real-time glucose levels from wearable sensors, and the points they have earned for their efforts. The app also sends timely encouragements to the participants to help them stay on track to complete the study.
Including participants as partners through virtual engagement
A core tenet of the Scripps Research Digital Trials Center is treating our study participants as partners. An important way we actualize this commitment is through our Virtual Advisory Teams (VAT), composed of dozens of community members who provide feedback on all aspects of our research. The two VATs we work with are our Digital Trials Center Virtual Advisory Team, and our All of Us Virtual Advisory team.
Our VAT members represent diverse communities, and their perspectives help our study teams engage with a broader spectrum of the public. They provide critical input on how research projects are conducted. Our studies benefit from the expertise and guidance of this group, which provides honest and open feedback on all aspects of our direct-to-participant studies, including program messaging, user experience, biosample collection, electronic health record retrieval and privacy/data security concerns. The VAT reminds us of the importance of designing studies with participants at the center. Learn more about our VAT and how to join here.

A note from our director of digital medicine
“20 years ago, the only way to find out something more about yourself is if you actually went to a clinic or when a doctor told you. But wearables and smartphones have completely changed that. Individuals can now collect so many biometrics about themselves, in their own lives. Our job, I see it now, as care providers, not just as physicians, but anyone who is providing care and looking out for someone’s health and well-being, is to make sense of all the data that’s being generated. And I think that’s where we can really make a difference. And that’s where medicine even, and wellness is going.” – Jay Pandit, MD



