Authors: Katie Quartuccio, Danielle Chiang and Jeff Pawelek –
The Scripps Research Digital Trials Center aims to gain valuable data from real life settings with minimal burden to study participants. As introduced in our previous post, our most intensive digital study to date, PROGRESS (PRediction Of Glycemic RESponse Study), examines individual glycemic responses. In preparation for this fully remote digital clinical trial, finding the most user-friendly, at-home blood draw device that could be used by people of all ages with varying physical and technical abilities was the primary goal.
To begin the search for the ideal at-home blood collection kit, we established the following core requirements to parse through the abundance of devices on the market:
- Class I or II exemption or Investigational Use status per FDA regulations
- Ability to test for Hemoglobin A1c (HbA1c)
- Ability to perform the entire collection at home without assistance
- Return shipping via a common provider with an easy pick-up option to return samples without leaving the home
- Wide range of collection hours and return days
Nine devices from six different companies initially met the core requirements. The devices offered a breadth of collection methods that include modalities such as finger prick collection and/or the use of a lithium heparin (LH) vial (Table 1). For the purposes of PROGRESS, we recognized that half of our cohort with type 2 diabetes may be accustomed to the finger prick method when using a glucometer; however, devices that leverage upper arm microneedle collection were preferred to minimize discomfort for all participants.
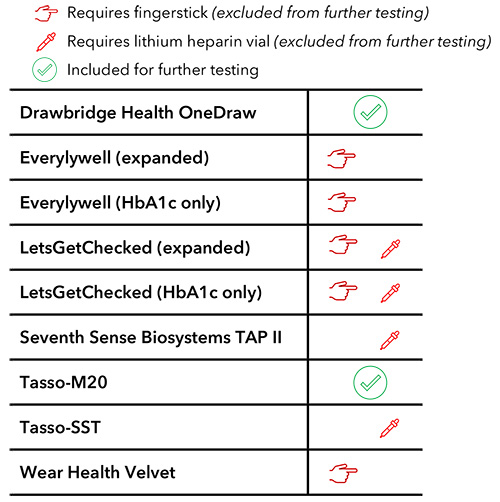
Table 1. Primary criteria checklist used to exclude blood draw devices from further testing.
If additional assays were in the scope of the study, the LH vials could provide more blood volume with a potential to process more analytes. However, additional analytes would require fasting and overnight shipping to the lab due to sample expiration. LH collection devices would restrict collection days to Monday through Wednesday morning, with prompt delivery to a postal carrier to ensure samples are received by the lab during business days/hours. We wanted to offer study participants the maximum flexibility regarding what days during the week they could ship their blood sample, so for each collection device we carefully examined the timeline of when the sample would be shipped, received, and processed. When selecting a device for a specific research need, the shipping conditions may be an important consideration.
The remaining two devices used dried blood cards that have fewer shipping restrictions and are collected via an upper arm microneedle. We evaluated the Drawbridge Health OneDraw (Figures 1a, 1b) and Tasso-M20 (Figures 2a, 2b) using secondary criteria with a focus on participant safety and ease of use that included: clarity of manufacturer instructions, complexity of the overall process, preparation for shipping, and degree of hand strength and dexterity necessary to perform the procedure. We drew comparisons between the two devices and how well they met the needs of the PROGRESS study protocol (Table 2).
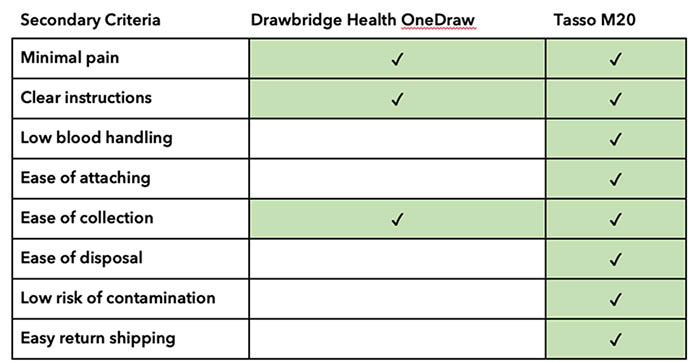
Table 2. Secondary criteria checklist used to evaluate safety and ease of use.
The OneDraw required significant finger strength to deploy the device and two buttons had to be pressed in the correct sequence while avoiding the potential for the device to slip off of the arm to activate the blood draw. After collection, the blood sample card enclosed in the device needed to be removed, which introduced direct exposure to the blood and the potential for spillage and contamination before being inserted into a plastic holding container for return shipment (Figures 1c, 1d). It was difficult to see how much blood was collected inside the device, so there was the potential to over/under fill the device. Therefore, having a second person to monitor the fill level or the use of a mirror may be needed to confirm the fill level. If a study coordinator was available for in-person or virtual assistance, the OneDraw would be a more feasible option for at-home blood collection without phlebotomy-trained personnel.
Additionally, depending on the participant’s local regulations, they would need to properly dispose of the device at a sharps disposal location. With regards to processing, a third-party lab capable of extracting the blood sample from the device would also be required.
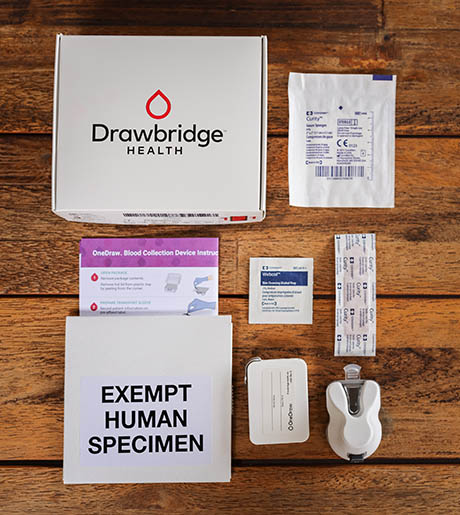
Fig 1(a). The Drawbridge Health OneDraw kit.

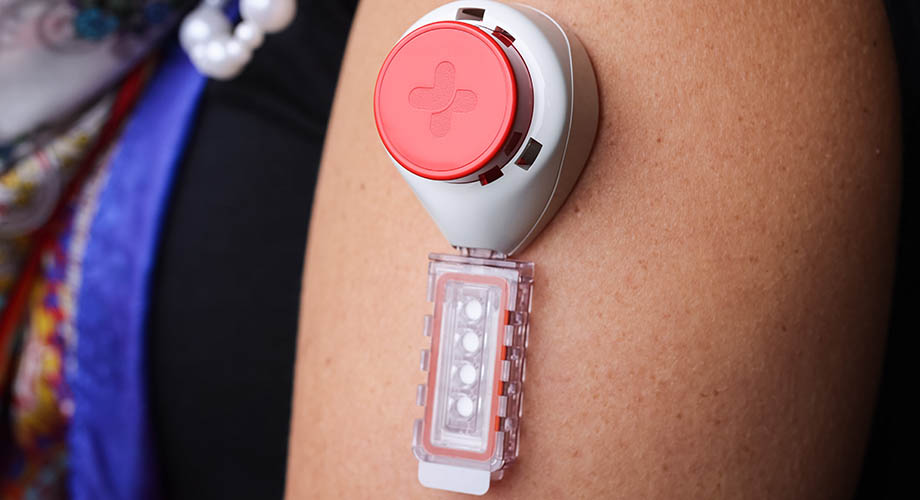
Fig 1(b). The OneDraw device affixed to the upper arm.

Fig 1(c). Removing the OneDraw cartridge from the device once blood collection is complete.

Fig 1(d). Placing the OneDraw cartridge into a second collection device in preparation for shipping.
Overall, the Tasso M-20 offered additional participant-friendly features that were closely aligned with a blood draw best suited for the self-guided PROGRESS experience. Once the blood draw was complete the entire collection device was placed into a biobag and returned to the company’s processing lab in the original shipping package, requiring fewer steps to complete the sample collection and preparation for return shipping. The self-contained unit also removed the burden of having to properly dispose of the microneedle device into an approved sharps container. Processing of the blood sample was facilitated by Tasso removing the additional requirement of identifying a third-party service. In summary, the M-20 was best aligned with the established study goals and participant experience envisioned for PROGRESS.

Fig 2(a). The Tasso M-20 kit.
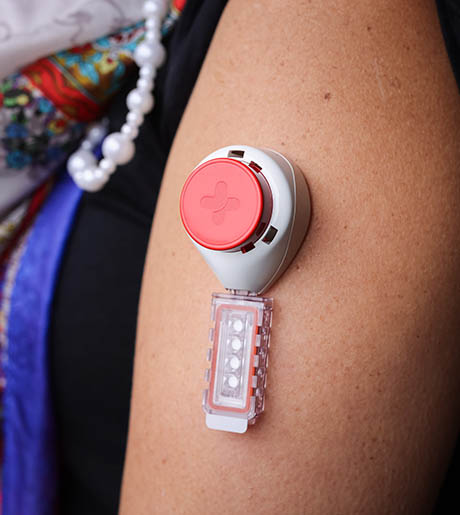
Fig 2(b). The Tasso M-20 device affixed to the upper arm.
The limitations of this review include the accuracy of test results, which were not independently analyzed and compared across the devices. Our goal was to develop and apply criteria in order to expedite a rapid assessment of blood draw devices. Therefore, this review should be viewed as an ad hoc summary. Finally, the selection of the Tasso M-20 was determined to be best suited for the PROGRESS study protocol; other devices with different feature sets may be more appropriate depending on the specific research needs.
Disclaimer: Drawbridge Health, Tasso, Seventh Sense Biosystems, and Weavr Health provided free sample kits for internal staff testing. All other devices used for internal testing and for the PROGRESS study were purchased with no additional financial incentives.